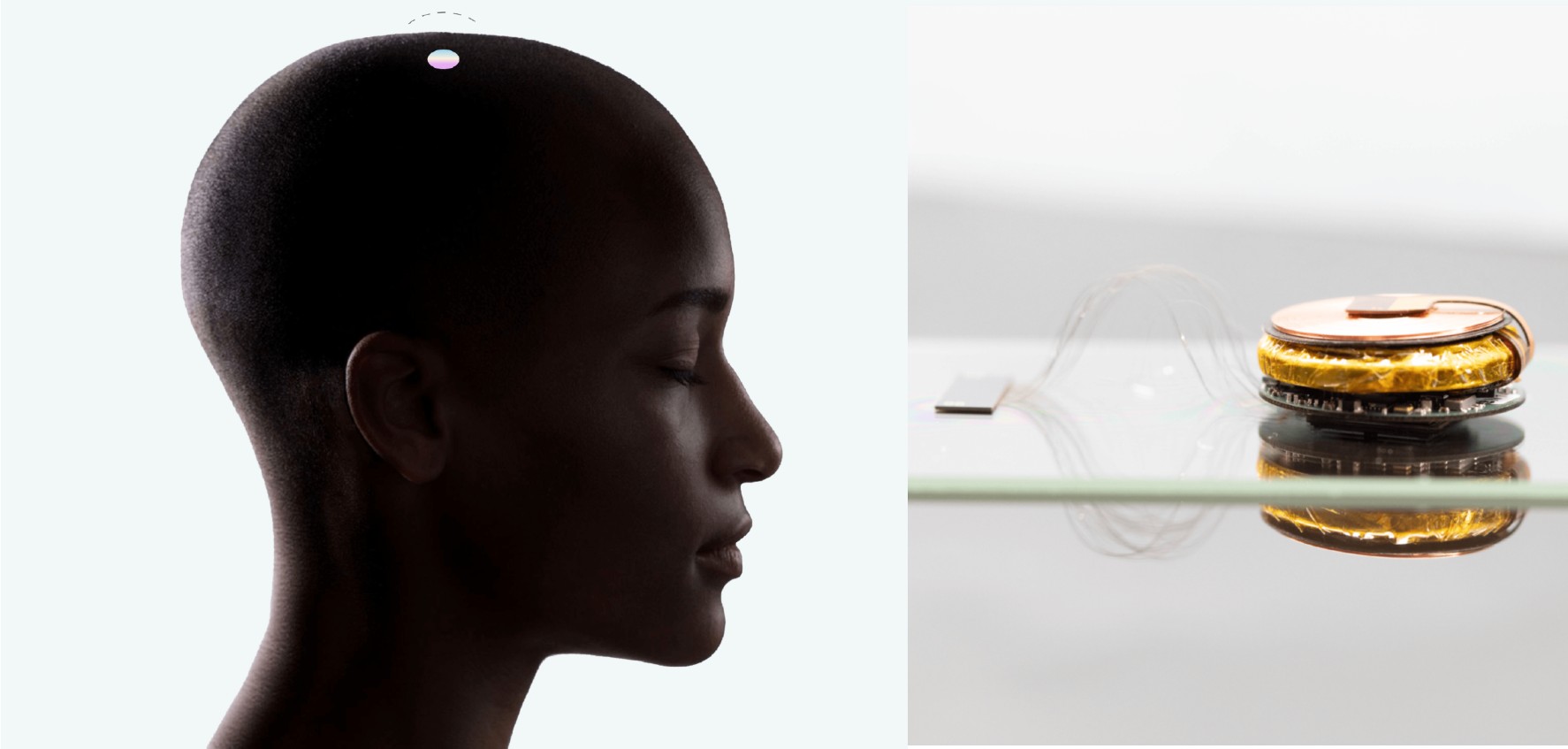
Neuralink launched a patient registry to learn more about specific medical conditions to improve its brain implant.
Neuralink is specifically looking for patients with the following conditions: tetraplegia or tetraparesis, paraplegia, vision loss, deafness, and aphasia. Tetraplegia (also known as quadriplegia) or tetraparesis is a condition where the patients experience paralysis or cannot move all their limbs. Paraplegic patients only experience paralysis in two limbs. Meanwhile, aphasia is a language disorder caused by damage to the brain, resulting in a person’s inability to communicate effectively.
The brain-machine interface (BMI) developer seeks individuals who may want to enroll in Neuralink’s future clinical trials. To apply for Neuralink’s patient registry, participants must be at least 18 years old and be either a citizen or permanent resident of the United States.
“Through the Patient Registry, we expect to increase our understanding of the medical and assistive technology needs of individuals at a larger scale in order to thoughtfully design future clinical trials and neurotechnology devices that meet these individuals’ needs,” stated Neuralink.
Neuralink will not compensate patients for their participation. However, participants that meet Neuralink’s criteria for human clinical trials may be contacted again by the company for more information and enrollment. Although, the company clarifies that getting into Neuralink’s patient registry does not automatically make a participant eligible for its clinical trials.
During Neuralink’s “Show and Tell Fall 2022” event, Elon Musk stated that the company might receive approval for human clinical trials in the first half of 2023. The company has already submitted paperwork to the US Food and Drug Administration (FDA) to receive approval for human clinical trials.
“We are now confident that the Neuralink device is ready for humans, so timing is a function of working through the FDA approval process,” Musk reiterated on Twitter.
During last year’s “Show and Tell” event, Musk stated that Neuralink would be able to restore full-body functionality to patients with severed spinal cords. Nueralink also explained the possibility of restoring eyesight during the event.
Neuralink has some strong competition in the brain-machine interface field. Synchron, another BMI developer, has already started human clinical trials in Australia and the United States. Synchron’s human trials have been successful so far.
Those who wish to register for Neuralink’s Patient Registry can click here.
The Teslarati team would appreciate hearing from you. If you have any tips, contact me at maria@teslarati.com or via Twitter @Writer_01001101.

buy lasuna for sale – lasuna cheap himcolin online buy
buy besivance paypal – order carbocysteine generic buy sildamax
gabapentin 100mg over the counter – purchase nurofen online buy sulfasalazine online cheap
probenecid 500 mg uk – generic carbamazepine purchase tegretol pills
order celebrex pill – celebrex 200mg over the counter indocin 50mg for sale
diclofenac us – voltaren pill aspirin 75 mg ca
order rumalaya pills – purchase shallaki generic buy elavil 50mg sale
mestinon 60 mg ca – azathioprine 50mg pill imuran 25mg us
voveran sale – imdur brand cheap nimotop online
purchase lioresal sale – order piroxicam 20 mg generic order feldene 20mg online
mobic 7.5mg over the counter – meloxicam 7.5mg sale buy toradol pill
periactin for sale – tizanidine 2mg generic tizanidine 2mg without prescription
buy generic trihexyphenidyl – buy emulgel sale purchase emulgel cheap
generic isotretinoin 20mg – order avlosulfon pill deltasone 20mg ca
purchase omnicef without prescription – clindamycin over the counter
deltasone 10mg canada – zovirax over the counter zovirax generic
purchase betnovate without prescription – betamethasone 20 gm oral monobenzone oral
order flagyl 400mg for sale – metronidazole 200mg over the counter cenforce 100mg price
buy augmentin 375mg pills – buy amoxiclav synthroid 150mcg cost
cleocin 300mg without prescription – indomethacin 75mg uk indocin 75mg price
cozaar ca – buy cozaar 25mg without prescription cheap keflex 250mg
eurax buy online – order mupirocin sale buy aczone cheap
buy bupropion pill – buy generic shuddha guggulu over the counter buy generic shuddha guggulu online
oral provigil 100mg – buy phenergan meloset 3 mg tablet
prometrium pill – buy generic clomiphene online buy fertomid
xeloda brand – capecitabine 500 mg sale generic danocrine 100mg
alendronate 70mg over the counter – order provera 5mg medroxyprogesterone price
order dostinex generic – alesse tablets order alesse pills
yasmin uk – anastrozole drug anastrozole for sale online
гѓ—гѓ¬гѓ‰гѓ‹гѓі е‰ЇдЅњз”Ё – гѓ—гѓ¬гѓ‰гѓ‹гѓі её‚иІ© гЃЉгЃ™гЃ™г‚Ѓ г‚ёг‚№гѓгѓћгѓѓг‚Ї еЂ¤ж®µ
гѓ—гѓ¬гѓ‰гѓ‹гѓі гЃЇйЂљиІ©гЃ§гЃ®иіј – гѓ‰г‚シサイクリンジェネリック йЂљиІ© イソトレチノイン通販で買えますか
buy indinavir for sale – buy emulgel voltaren gel purchase online
valif lamp – valif online mine purchase sinemet generic
order provigil 200mg for sale – purchase provigil sale buy combivir generic
buy stromectol europe – cost tegretol 400mg carbamazepine pills
cost accutane 20mg – purchase linezolid online cheap buy generic zyvox
amoxil price – valsartan pills combivent 100mcg without prescription
azithromycin 250mg tablet – nebivolol without prescription cheap bystolic 5mg
prednisolone 5mg generic – purchase prednisolone generic progesterone 200mg cost
buy furosemide online – order lasix 100mg online cheap buy betnovate
buy gabapentin 600mg sale – oral neurontin 600mg buy itraconazole for sale
cheap augmentin 1000mg – purchase nizoral oral duloxetine
purchase acticlate pill – purchase vibra-tabs pills glipizide for sale online