High-nickel-content cathodes have captured the imagination of scientists, as they could enable much higher energy density. However, the high nickel content causes these cathode materials to degrade more quickly, creating cracks and stability issues as the battery cycles.
A team of researchers at the DOE’s Brookhaven National Laboratory investigated the valence gradient of cathode materials in order to understand its effect on battery performance. Their findings, published in Nature Communications, demonstrate that the valence gradient can serve as a new approach for stabilizing the structure of high-nickel-content cathodes against degradation and safety issues.
Scientists have synthesized materials made with a nickel concentration gradient—that is, the concentration of nickel gradually changes from the surface of the material to its center, or bulk. These materials have exhibited greatly enhanced stability, but scientists have not been able to determine if the concentration gradient alone was responsible for the improvements. The concentration gradient has traditionally been inseparable from another effect called the valence gradient, or a gradual change in the nickel’s oxidation state from the surface of the material to the bulk.
In the new study, chemists synthesized a novel material that isolated the valence gradient from the concentration gradient.
“We used a unique material that included a nickel valence gradient without a nickel concentration gradient,” said Brookhaven chemist Ruoqian Lin, first author of the study. “The concentration of all three transition metals in the cathode material was the same from the surface to the bulk, but the oxidation state of nickel changed. We obtained these properties by controlling the material’s atmosphere and calcination time during synthesis. With sufficient calcination time, the stronger bond strength between manganese and oxygen promotes the movement of oxygen into the material’s core while maintaining a Ni2+ oxidation state for nickel at the surface, forming the valence gradient.”
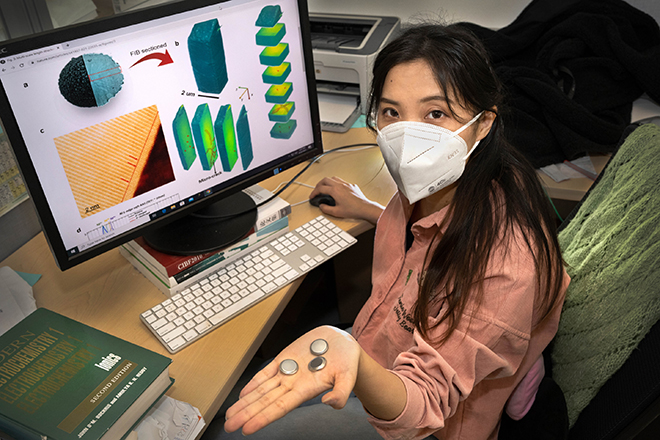
The Brookhaven researchers studied the new material’s performance using two cutting-edge experimental tools: the Hard X-ray Nanoprobe (HXN) beamline and the Full Field X-ray Imaging (FXI) beamline. By combining the capabilities of both beamlines, they were able to visualize the atomic-scale structure and chemical makeup of their sample in 3D after the battery operated over multiple cycles.
“Both beamlines have world-leading capabilities. You can’t do this research anywhere else,” said Yong Chu, leader of the imaging and microscopy program at NSLS-II and lead beamline scientist at HXN. “FXI is the fastest nanoscale beamline in the world; it’s about ten times faster than any other competitor. HXN is much slower, but it’s much more sensitive—it’s the highest resolution X-ray imaging beamline in the world.”
HXN beamline scientist Xiaojing Huang added, “At HXN, we routinely run measurements in multimodality mode, which means we collect multiple signals simultaneously. In this study, we used a fluorescence signal and a phytography signal to reconstruct a 3D model of the sample at the nanoscale. The florescence channel provided the elemental distribution, confirming the sample’s composition and uniformity. The phytography channel provided high-resolution structural information, revealing any microcracks in the sample.”
Meanwhile at FXI, “the beamline showed how the valence gradient existed in this material. And because we conducted full-frame imaging at a very high data acquisition rate, we were able to study many regions and increase the statistical reliability of the study,” Lin said.
At the CFN Electron Microscopy Facility, the researchers also used an advanced transmission electron microscope (TEM) to visualize the sample with ultra-high resolution. By combining the data collected across all of the different facilities, the researchers were able to confirm that the valence gradient played a critical role in battery performance. The valence gradient “hid” the more capacitive but less stable nickel regions in the center of the material, exposing only the more structurally sound nickel at the surface. This arrangement suppressed the formation of cracks.
“These findings give us very important guidance for future novel material synthesis and design of cathode materials, which we will apply in our studies going forward,” Lin said.
Source: Phys.org
Your perspective on [topic] is refreshing. Keep these posts coming!
This is one of the best explanations I’ve come across. Thanks!
lasuna sale – buy diarex sale cheap himcolin without prescription
I enjoyed reading this. It’s clear and well-written.
buy besivance generic – buy generic sildamax for sale buy sildamax paypal
gabapentin over the counter – azulfidine usa order azulfidine 500 mg
probenecid 500 mg uk – tegretol brand tegretol 200mg usa
purchase colospa sale – mebeverine price generic pletal 100 mg
order celecoxib 200mg generic – buy cheap celebrex indomethacin us
cost diclofenac 100mg – brand voltaren aspirin 75 mg usa
purchase rumalaya pills – endep price amitriptyline oral
buy pyridostigmine 60 mg sale – how to buy pyridostigmine order generic imuran 25mg
buy diclofenac cheap – purchase nimotop sale nimodipine generic
order ozobax for sale – ozobax ca feldene oral
cheap meloxicam 7.5mg – rizatriptan 10mg uk buy ketorolac pills for sale
buy cyproheptadine tablets – order periactin 4 mg generic where can i buy zanaflex
purchase artane without prescription – buy diclofenac gel online order diclofenac gel
buy omnicef 300mg for sale – omnicef 300mg pills buy generic clindamycin online
buy cheap generic isotretinoin – order generic aczone deltasone 5mg cheap
order permethrin online cheap – oral tretinoin cream retin cream for sale
purchase betnovate sale – benoquin price order monobenzone generic
metronidazole 200mg us – buy metronidazole 400mg generic buy generic cenforce for sale
augmentin oral – levothroid price cost synthroid 75mcg
purchase cleocin without prescription – indomethacin cheap order indocin for sale
losartan order online – generic cephalexin 250mg buy keflex 125mg
where can i buy crotamiton – crotamiton sale how to buy aczone
order provigil online – promethazine 25mg pill purchase melatonin
buy zyban generic – buy ayurslim without a prescription shuddha guggulu oral
buy prometrium pills for sale – order progesterone 100mg clomiphene medication
capecitabine brand – order xeloda 500 mg without prescription buy danocrine without prescription
buy cabergoline paypal – order cabgolin generic alesse without prescription
yasmin price – buy ginette 35 cheap buy anastrozole without prescription