At the Osaka Prefecture University, Atsushi Sakuda and colleagues have been studying a type of battery that they believe holds the key to the future of energy storage: all-solid-state batteries (ASSBs).
“In 2019, we developed a solid electrolyte using sodium ions, which showed the highest conductivity in the world reported at room temperature,” said Dr. Sakuda. The team’s latest study, published in Science Advances, details a method for developing new electrode materials for safe and efficient ASSBs.
The non-flammable nature of solid electrolytes is expected to make ASSBs safer than traditional Li-ion batteries. It is also possible to miniaturize these cells, as they do not require separators or cooling systems.
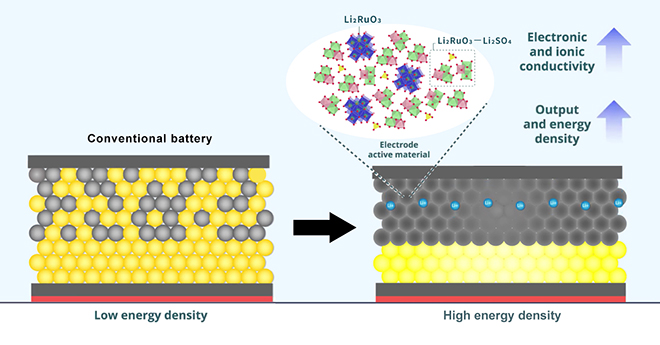
However, one obstacle remains: it is difficult to achieve effective contact between the electrolyte and the electrode active material, and this decreases the battery’s energy density and performance. “Finding novel, efficient electrode materials is key for manufacturing ASSBs with high energy density,” said Dr. Sakuda.
To solve this problem, the researchers developed a positive electrode material by combining two lithium compounds: lithium sulfate (Li2SO4) and lithium ruthenate (Li2RuO3). The resulting matrix provided more space for ions to flow through, enabling a faster transfer of charge. The addition of Li2SO4 also made the overall structure more “ductile” and “amorphous,” or easy to mold, which enables a reversible redox reaction and allows for the matrix to be further compressed to increase electronic and ionic conductivity and stability.
The researchers say the batteries’ “reversible” capacity (the ability to charge and re-charge) of 270 mAh/g outperforms most previous ASSBs. They plan to swap out the expensive ruthenium (Ru) element in the electrode with another, cheaper metal with similar properties. That being said, they believe that their method provides a solid basis for the manufacture of next-generation batteries.
Source: Asia Research News